About 20% females are diagnosed with a chronic pelvic pain related disorder (CPPD), such as endometriosis, adenomyosis, and uterine fibroids. The condition affects their work productivity and quality of life. The type and intensity of symptoms experienced vary between patients and there are daily fluctuations in symptoms. Currently there are no approved surveys or questionnaires to assess the status of the condition or to routinely monitor treatment progress or outcomes. CPP Tracker is a research study that aims to develop digital patient-reported outcome measures (PROMs) for CPPDs that can capture the multi-dimensionality of pain symptoms and can be used for routine outcome monitoring. If you have a diagnosis for a CPPD (e.g., endometriosis, adenomyosis, fibroids) and have experienced pelvic pain for at least 6 months, you might qualify to participate in CPP Tracker.
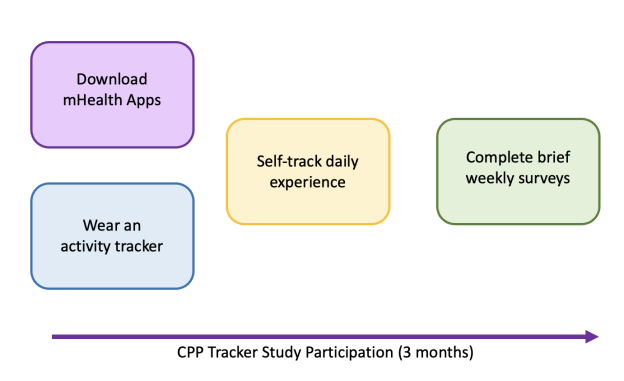
Participation in the study involves 3 months of self-tracking daily symptoms, well-being, self-management techniques using a research mobile phone App, and wearing an activity tracker (Fitbit Inspire 3) provided by the study team. If you choose to participate in this study, you will be asked to:
Download two mHealth apps, Phendo and eHive
Use Phendo to self-track your daily symptoms, well-being, self-management techniques
Complete brief standardized questionnaires on pain and quality of life each week through the eHive app
Wear an activity tracker on your wrist every day for 3 months
The data from the apps and the Fitbit will be used to identify factors that can help suggest if a treatment is working (e.g., Does physical therapy/medication/meditation help with pain symptoms?). The data will also be used to develop clinical measures that allow providers to capture the disease experience and wellbeing of their patient more comprehensively. In the long term, implementation of these tools in clinical settings can help CPP patients manage their condition and help clinicians make more informed treatment decisions.
To read more about the study and see if you are eligible to participate, you can scan the QR code or click here.

CPP Tracker is being conducted by our research team at the Hasso Plattner Institute for Digital Health at Mount Sinai and the Department of Artificial Intelligence and Human Health within the Icahn School of Medicine at Mount Sinai (Principal Investigator: Ipek Ensari, PhD). CPP Tracker is approved by the Mount Sinai Institutional Review Board with all data securely maintained in the Mount Sinai system. Your privacy is our top priority.
For questions regarding study participation, you can reach out to our study team at ehivecpp@mssm.edu.






